SynaptixBio awarded second FDA Orphan Drug Designation to boost search for rare disease therapies
- Dr Dan Williams

- Apr 29, 2024
- 3 min read
SynaptixBio now aiming to develop treatment for second variant of TUBB4A leukodystrophy
A leading biotech firm working to develop the world’s first treatment for a rare, incurable and deadly disease has secured a second Orphan Drug Designation (ODD) from the US Food and Drug Administration (FDA).
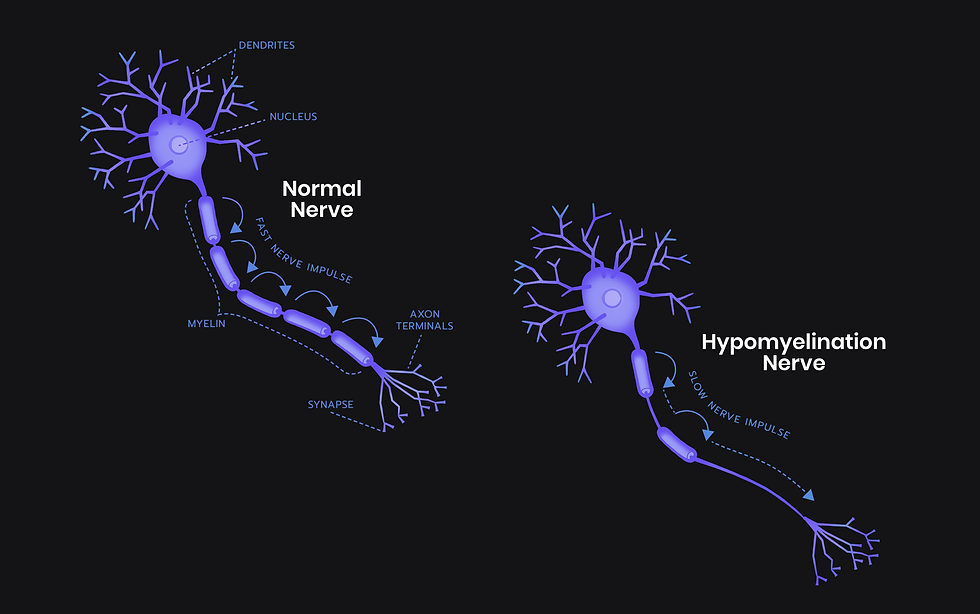
SynaptixBio received its first ODD in early 2023 for a therapeutic that targets Hypomyelination with Atrophy of the Basal ganglia and Cerebellum (H-ABC), the most severe form of TUBB4A leukodystrophy.
This second ODD allows research and development of a therapy for another form of the disease, Isolated Hypomyelination.
Dan Williams, CEO at SynaptixBio, said; “This ODD is a huge boost to our efforts in tackling these devastating, life-limiting rare diseases. Our whole strategy is centred around achieving these prestigious designations, which make development of therapeutics easier and more cost-effective.”
Isolated Hypomyelination was identified relatively recently and little is known about it generally. It appears to be like H-ABC but without atrophy of the basal ganglia and cerebellum, so effectively the H without the ABC. The symptoms are reported to be milder than H-ABC.
SynaptixBio was late last year awarded a £490,000 BioMedical Catalyst grant from Innovate UK specifically to tackle less common variants of the disease, so this ODD award represents the next step in their search for therapies.
Earlier last year SynaptixBio successfully led a second round of investment, taking the total up to £13.2m, which will take it up to the start of in-human clinical trials later this year.
An ODD enables firms to cut research costs through tax credits, secure grants to offset development expenses, and gain exemption from some pre-marketing authorisation requirements and regulatory fees. In addition, it grants after approval a potential seven years of market exclusivity and data exclusivity.
This second ODD designation follows the award late last year of a Rare Paediatric Disease Designation (RPDD), which can lead to the award of a Priority Review Voucher (PRV) once a product is approved.
A PRV can expedite the FDA’s product review time, and it can be sold or transferred, for example to one of the big pharmaceutical companies (Big Pharma), which can potentially offset the high costs associated with the development of rare disease therapies.
Rare diseases predominantly have a genetic origin, affect the young, and are very often life-limiting, so the impacts on families are devastating and enormous.
SynaptixBio is using antisense oligonucleotide (ASO) technology to tackle TUBB4A-related leukodystrophies; ASOs can alter the expression of genes, in this case a specific ASO molecule targets the mutated TUBB4A gene to stop it forming toxic proteins, which in turn help build the cells that form myelin sheaths surrounding nerve fibres in the brain. With the toxic protein suppressed, other proteins step in to help form normal myelin.
The technology has been proven in the treatment of other dystrophies, including Duchenne muscular dystrophy, and is quick and cost-effective to develop.
According to a European Commission report from 2020, “1 in 17 people will be affected by a rare disease at some point in their lives. This amounts to 3.5 million people in the UK”.
There are over 7,000 known rare diseases, with more emerging all the time.
Disease research is carried out by the world's leading centre for leukodystrophy studies, the Children's Hospital of Philadelphia (CHOP), under a sponsored research agreement.
SynaptixBio has signed a worldwide exclusive license to intellectual property from CHOP, enabling commercialisation of a treatment.
Links to associated articles:




Comments